Guide to the Center
Welcome to the Daegu-Gyeongbuk Medical Cluster, the future of the global medical industry!
A trusted and dependable strategic partner for your
business collaborations!
Providing Comprehensive Services for Medical
Device Development
We support the development of image-based diagnostic convergence
technologies and IT-based therapeutic devices through:
- Commercialization support and one-stop service solutions focused on three major disease areas: cardiovascular and cerebrovascular diseases, geriatric diseases, and cancer
- Demand-driven collaborative clinical research tailored to real-world medical industry demands
About The Medical Device Development Center
Purpose of establishment
To overcome existing limitations in the medical device industry and enhance the successful commercialization of medical devices by building a globally competitive R&D infrastructure.
Vision
To identify and develop breakthrough technologies with and on behalf of partner companies, foster a virtuous cycle of technology discovery, business creation, and reinvestment, and establish Korea as a global hub for the IT-based medical device industry.
Major functions: R&D support for the commercialization of IT-based medical devices
- R&D Support for Commercialization of IT-Based Medical Devices : Comprehensive support throughout the development lifecycle to accelerate market entry
- Research Equipment and Facilities : Access to cutting-edge convergence imaging systems and testing equipment for performance and reliability evaluation
- Technology Verification and Reliability Testing : In-depth product analysis and application-based technology testing to ensure technical reliability
- R&D Assistance : Support for improving product performance and reliability, leading to successful commercialization
- Industry–Academia–Research Collaboration : Facilitation of cooperative networks and support for bridging technology gaps through targeted joint efforts
From Idea to Market, a seamless pathway encompassing:
Idea exploration → Collaborative R&D → Prototyping → Product testing → Pre-clinical trials → Clinical application → Market
Offering one-stop solutions for the end-to-end development of medical devices
Special advantages of the Medical Device Development Center
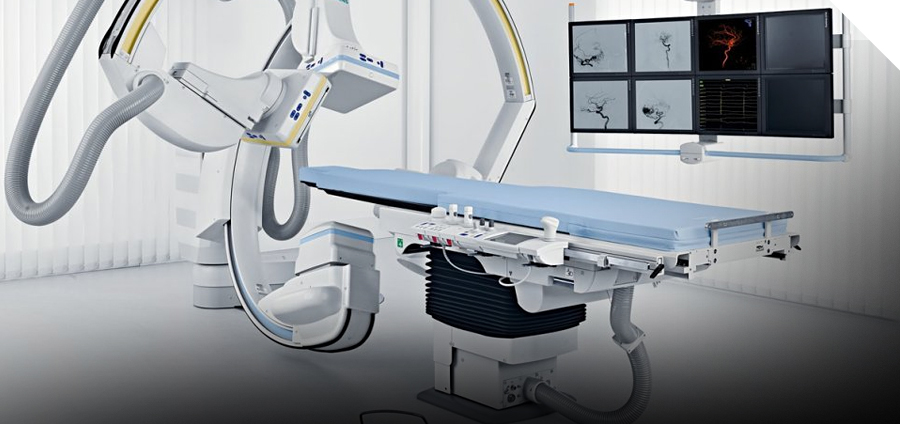
- Specialized Medical Imaging Systems : Home to the world’s first imaging infrastructure dedicated to medical device development, including MRI, Angiography, and PET-CT systems.
- Electromagnetic Compatibility (EMC) Testing : Comprehensive EMI and EMS testing facilities, including 10m/3m chambers and certified EMS systems (KOLAS accredited), to ensure compliance and safety.
- Certified Medical Device Testing Institution : Officially designated by the Ministry of Food and Drug Safety for electrical, mechanical, and reliability testing of medical devices.
- Prototype Manufacturing Capabilities : In-house support for PCB fabrication, SMT packaging, and precision component processing.
- Industry Support Programs : Tailored corporate support through one-on-one mentoring and field-specific training programs.
Floor plan
- HVAC and Electrical Systems, GPU Server room 5F
- Administrative area 4F
- R&D labs and facilities 3F
- R&D labs and facilities , Medical Device Safety assessment
facilities and Administrative area 2F - Human-Driven material research lab and Administrative area1F
- Electromagnetic Chamber, GLP(Good Laboratory Practice) facilities, CNC Precision Machining, Prototyping manufacturing facilities, B1