Guide to the Center
Welcome to the Daegu-Gyeongbuk Medical Cluster, the future of the global medical industry!
A strong and reliable strategic partner
for collaborating businesses!
Offer comprehensive services for
the development of medical devices
Support for the development of image-based convergence diagnostics and
IT-based treatment devices
- Support for commercialization and one-stop service solutions focusing on three major types of diseases: cardiovascular/cerebrovascular diseases, geriatric diseases, cancer)
- Demand-oriented joint clinical research
About the New Drug Development Center
Purpose of establishment
Overcoming limitations within the medical device industry and improving the successful commercialization of medical devices through the establishment of an R&D infrastructure on a global scale.
Vision
Discover leading technologies on behalf of partner companies, create a virtuous cycle (technology exploration, business creation, and reinvestment), and build a global hub for the IT-based medical devices industry in Korea
Major functions: R&D support for the commercialization of IT-based medical devices
- Research equipment support: convergence imaging equipment, research equipment (for performance and reliability assessments)
- Technology reliability: product technology analysis and technology application testing
- R&D support: performance and reliability improvement, product commercialization
- Industry–university–research network: support for vacant technology
Idea exploration–collaborative R&D–prototyping–product testing–pre-clinical trials–application to clinical practice
Provision of one-stop solutions for the development of medical devices
Special advantages of the New Drug Development Center
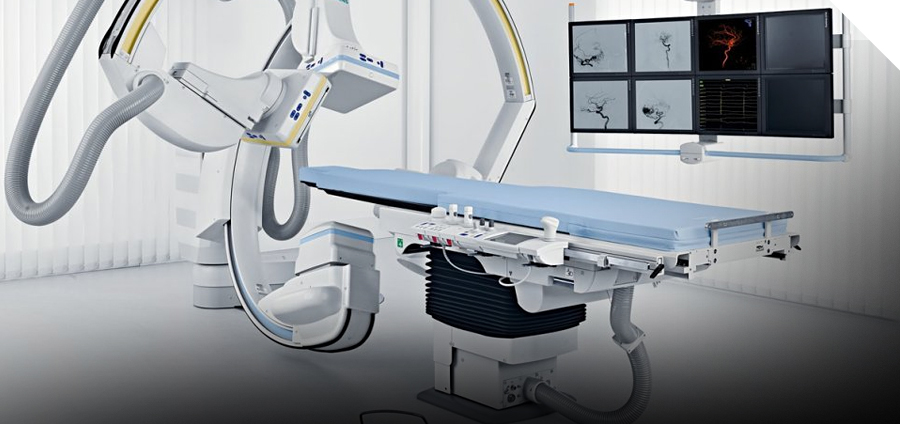
- Medical imaging devices: the world’s first medical imaging system specifically designed to assist in the development of medical devices (MRI system, Angio system, PET-CT system)
- Electromagnetic compatibility: electromagnetic interference testing of medical devices (10/3M chamber, EMS chamber, EMI & EMS system (KOLAS certification))
- Medical device inspection institution: designated an official testing institution by the Ministry of Food and Drug Safety for the electrical and mechanical performance testing and reliability testing of medical devices
- Prototype manufacturing: PCB manufacturing, SMT packaging, precision processing devices Corporate support program: one-to-one mentoring and education by field
Floor plan
- Air conditioning and heating room, fan room 5F
- R&D room, collaborative research room: 3F-4F
- Safety assessment, rental lab 2F
- Administrative office, business support office, electromagnetic testing room 1F
- Micro precision processing room, prototype manufacturing room (MRI) B1
- Electromagnetic chamber (electromagnetic certification & testing facilities),
biological testing room, machines & electricity room B2