Core capabilities
Welcome to the Daegu-Gyeongbuk Medical Cluster, the future of the global medical industry!
Core capabilities

Prototype design support
- PCB manufacturing and SMT packaging
- Device design services
- Precision processing
- Education for prototype manufacturing and medical device characterization

Technological support for diagnostic and treatment devices
- Imaged-based high-intensity focused ultrasound devices
- Ultrasound medical device performance evaluation devices
- Performance tests for ultrasound medical devices to obtain Ministry of Food and Drug Safety certification and CE licensing

In vitro technology support
- In vitro diagnostic device performance testing equipment
- In vitro diagnostic production development support
- Design of core platforms for molecular diagnosis
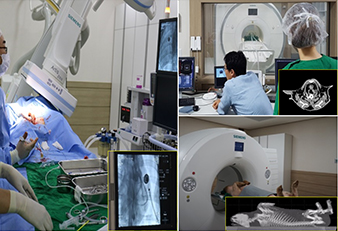
Medical imaging technology support
- Development of a medical imaging system based on high-tech medical imaging devices (MRI, PET-CT, x-ray angiography)
- Collaborative development of medical devices through the use of high-tech medical imaging equipment
- Pre-clinical testing to assess the efficacy and safety of medical devices
- Development of animal models for specific diseases
- Support for research on brain functioning

Robot Device Team
- Technological support for medical robotic devices
- Support for robot-assisted surgery development platforms
- Support for medical simulators
- Medical image registration and signal processing technology

Testing for medical device certification
- Certified testing institution designated by the Ministry of Food and Drug Safety for the issuance of test certificates for licenses and permits
- - Surgical devices, diagnostic devices, medical stimulators, in vitro diagnosis devices, syringes and needle for syringes, ubiquitous healthcare medical device
- Electromagnetic wave testing institution certified by KOLAS
- Support for device reliability analysis using large environmental chambers and highly accelerated life testing devices
- Certified testing institution designated by the Ministry of Food and Drug Safety for the issuance of test certificates for licenses and permits
02.
Medical Device Development Center
