Project Overview & Procedure
Welcome to the Daegu-Gyeongbuk Medical Cluster, the future of the global medical industry!
OASIS Project (company support program)
※ Open-Analysis, Solid and Infection drug Serviec
- Much like an oasis in the middle of a desert, the OASIS Project aims to quench companies’ thirst for the technologies they need to further their business endeavors.
Scope of Support
- Non-clinical trials
- Investigational new drug application (IND)
- Clinical trials (Phase I-III)
- New drug application (NDA)
- Permission/sales
- Manufact
uring- Manufacture and supply of medicine and medical supplies (drug products and pharmaceutical ingredients)
- Process development
- Formulation study
- Analysis
- Support for the development of analytical methods
- Stability testing (determination of expiration dates and retest periods, conducting of on-going stability tests)
- Quality control (QC)
- Chemistry, manufacturing, and control (CMC) support
Areas of Support
Support for the manufacturing of medicine and medical supplies
- Contract manufacturing of R&D medicine and medical supplies, joint utilization of facilities
Scope of Support
| Category | ||
|---|---|---|
| Solids (except cytotoxic drugs) View PDF | Tablets (single/multi-layered tablets) | |
| Capsules | ||
| Injections (cytotoxic drugs) View PDF | Liquids | Vials (6 ml, 15R, 20R, etc.) |
| Freeze-dried drugs | ||
| Pharmaceutical ingredients (except cytotoxic drugs) View PDF |
Reactors (10L, 100L, and 150L) | |
Analysis Support
- Support for the analysis of ingredients and drug products in the development stage, development of analytical methods, validation, and stability testing
Scope of Support
- Development of analytical methods, stability testing, lot release tests, etc.
GMP consulting, support for permits/licensing
- Provision of consultations for GMP facilities and the manufacture and analysis of drugs, support for filing permit/licensing applications and monitoring the progress of these applications
Scope of Support
- Drug production and processing, analysis techniques, environmental health and safety (EH&S), establishment, operation, and management of GMP facilities, investigational new drug application (IND), new drug application (NDA), etc.
Support Procedure
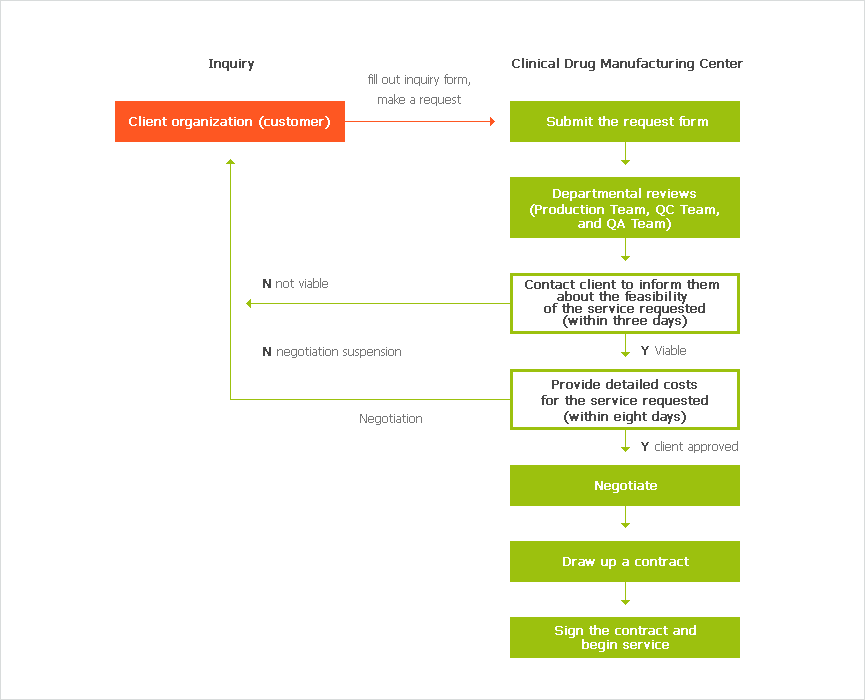
※ To make an inquiry, please fill out the inquiry form on our website (www.kmedihub.re.kr), and contact us by either phone or email.
(Tel) +82-53-790-5877 (E-mail) cdmc@kmedihub.re.kr
Professional Personnel Development Project
Purpose
To provide GMP training and practical training for students of pharmaceutical science programs and employees of organizations related to the pharmaceutical industry through the use of the facilities, equipment, and human resources at the Clinical Drug Manufacturing Center
Eligibility
- Employees of organizations related to the pharmaceutical industry
- Students in pharmaceutical science programs
Training Areas
- Production of medicine and medical supplies, such as solids, injections, and pharmaceutical ingredients
- Quality control and assessment
- Management and operation of GMP facilities
(Tel) +82-53-790-5877
